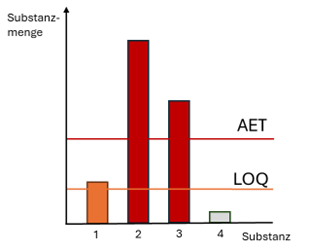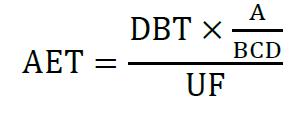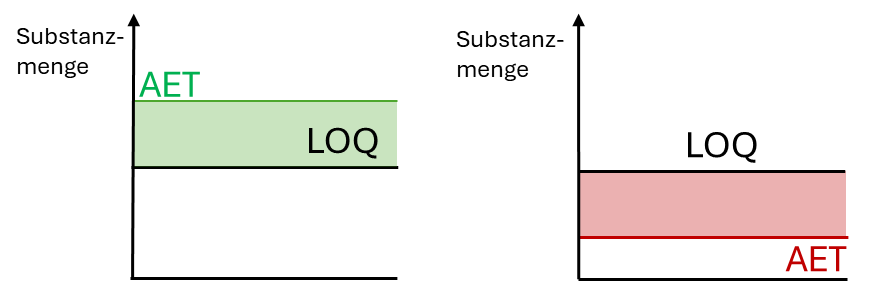Biocompatibility: Risks of incorrect AET determinations in chemical characterization and toxicological risk assessment
The chemical characterization according to DIN EN ISO 10993-18:2023-11 and the toxicological risk assessment (TRA) according to DIN EN ISO 10993-17:2024-02 are essential components of the biological evaluation of medical devices. A critical parameter in this process is the Analytical Evaluation Threshold (AET).
The correct setting of the AET is crucial to ensure that all relevant extractables & leachables are evaluated. This article highlights the potential consequences of incorrectly defining the relevant variables to calculate the AET.
Meaning of the AET
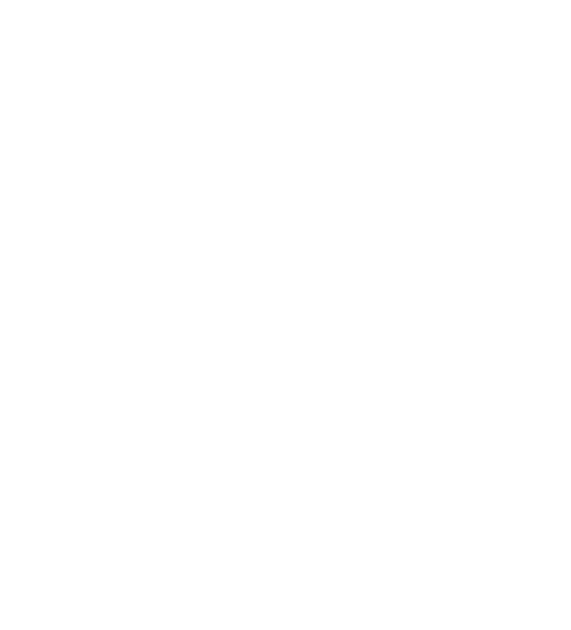
The AET (see Fig. 1) serves as a threshold value below which chemical compounds are considered toxicologically safe. Therefore, an evaluation up to the AET is sufficient in most cases. If substances of very high concern (SVHC) or cohorts of concern (CoC) are expected, these must be considered separately.
In order to be able to use the AET as an assessment limit, it is important that it is also technically feasible. Each laboratory has its own internally defined limits of quantification (LOQ), which must lie below the defined AET.
Fig. 1 shows the relationship between the AET and LOQ in the optimum case. Substances 2 and 3 (red) are above the AET and must therefore be evaluated and considered toxicologically. Substances 1 (orange) and 4 (grey), on the other hand, do not need to be considered. In this case, if the substances are CoCs or SVHCs, an evaluation would only be possible for substance 1, as substance 4 is below the LOQ and therefore cannot be quantified. Due to various restrictions and exceptions, this approach should be verified by a toxicologist.
Calculation of AET
The AET is calculated in accordance with DIN EN ISO 10993-18:2023-11 as shown in the formula (Fig. 2). The meaning of the individual parameters in the AET formula is explained below.
DBT - dose-based threshold value
The TTC (Threshold of Toxicological Concern) is often used for the DBT. This depends on the duration of use of the medical device (ISO/TS21726). The longer the duration of use, the lower this value is. Here too, a subsequent adjustment (reduction) can result in the AET falling below the LOQ.
A - Number of extracted medical devices
B - Extraction volume
DIN EN ISO 10993-12:2021-08 provides recommendations on which extraction ratio should be used for a medical device. The calculated volumes refer either to the surface area or the weight of the medical device. For certain geometries, it is necessary to increase the volume in order to cover the entire test specimen.
With a very small DBT or with very high volumes (B), e.g. due to very large test specimens or by increasing the volume due to the geometry, it is possible that the AET falls below the LOQ.
C - Number of medical devices usually used simultaneously per patient
This value should be determined on the basis of the actual application. Various examples are given below:
Retractor:
2 retractors are required to hold up a wound (C=2).
Dental implants:
Usually no more than 2 teeth are replaced with implants. (C=2).
As a worst-case scenario, a maximum of 4 teeth can be replaced with implants (C=4).
If these are replaced again after 10 years, for example, the C value is still 4.Catheter:
The catheter is changed every day, but only one catheter is used at a time (C=1).
If the C-value is corrected (increased) after the analyses have been performed, the AET can fall below the LOQ.
D - Concentration or dilution factor
Factor to consider the concentration (D<1) and dilution steps (D>1) performed during the analysis.
UF - Uncertainty factor
The evaluation using semi-quantification (calculation of the quantity using representative standards instead of exact quantification) may result in deviations from the actual value. This is due to the fact that each substance has a different response factor. This means that the integral of the peak in the chromatogram is not only influenced by the quantity, but also by interactions with the chromatography column or similar. With the help of the UF it is possible to take these fluctuations into account. This value is determined by the analysis method and cannot be influenced.
Problematic
The calculation of the AET can show two different scenarios (see Fig. 4). If the AET is above the LOQ, the method is sensitive enough to quantify toxicologically relevant substances (green). If, on the other hand, the AET is below the LOQ, there is a gap (red) in which the substances cannot be quantified with sufficient accuracy using the method.
Scenario 1: AET falls below LOQ due to adjustment
If the AET is set too high before the start of chemical characterization and has to be increased later as part of the toxicological risk assessment, the result may be that the extracts were not concentrated to a sufficiently high level. As a direct consequence, it cannot be ruled out that toxicologically relevant compounds could be detected and quantified. This has a negative effect on the toxicological risk assessment.
Scenario 2: AET increases due to adjustment
If the AET is set too low before the start of chemical characterization, it is possible to increase it subsequently as part of the toxicological risk assessment. The consequences are less problematic than if the AET is too low.
Preventive measures - Corrective measures
If it is clear in advance that the AET will fall below the LOQ, it is possible to take various corrective measures in advance. Possible corrective measures are:
- Concentration of the extracts (D<1)
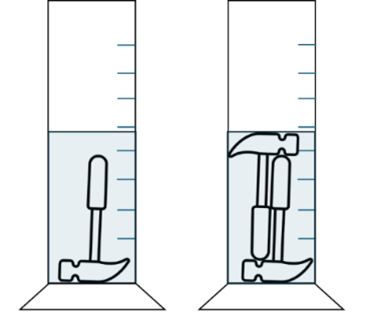
- Simultaneous extraction of several test items (pooling, A) to minimize the geometry-related increase in volume (Fig. 4)
However, given the high variability of medical devices, this is always a case-by-case decision. It is therefore particularly important to provide realistic and well thought-out information on the individual variables.
Conclusion
The correct determination of the AET is crucial for the accuracy of the chemical characterization and the toxicological risk assessment procedure. Setting the AET too low or too high can lead to significant problems that affect the evaluation of the chemical characterization in the biological assessment. It is therefore important to determine the AET carefully and precisely to ensure a comprehensive and reliable assessment. The test laboratory therefore relies on the manufacturer to address these criteria in advance and to specify the relevant variables C (number of medical devices used simultaneously) and DBT (dose-related threshold) directly and correctly with the testing order.
Newsletter registration