Decay measurement or double inspection - when does which qualification test make sense?
The qualification test according to VDA 19.1 / ISO 16232
The qualification test in accordance with VDA 19.1 / ISO 16232 is an essential step in determining the test parameters for the extraction processes and ensuring their effectiveness. The qualification test is intended to prove that detachable contaminants are extracted from the component as completely as possible. As the detachment of the particle load is strongly influenced by component-specific factors and extraction processes, there are no standardized test parameters. These must therefore be determined and verified by the qualification test.
Methods for qualification are decay measurement and, optionally in certain cases, the double inspection. In both cases, this involves repeated sampling of a component to determine the decay behavior. This white paper compares the decay measurement and the double inspection with regard to their implementation, interpretation and areas of application.
Decay measurement
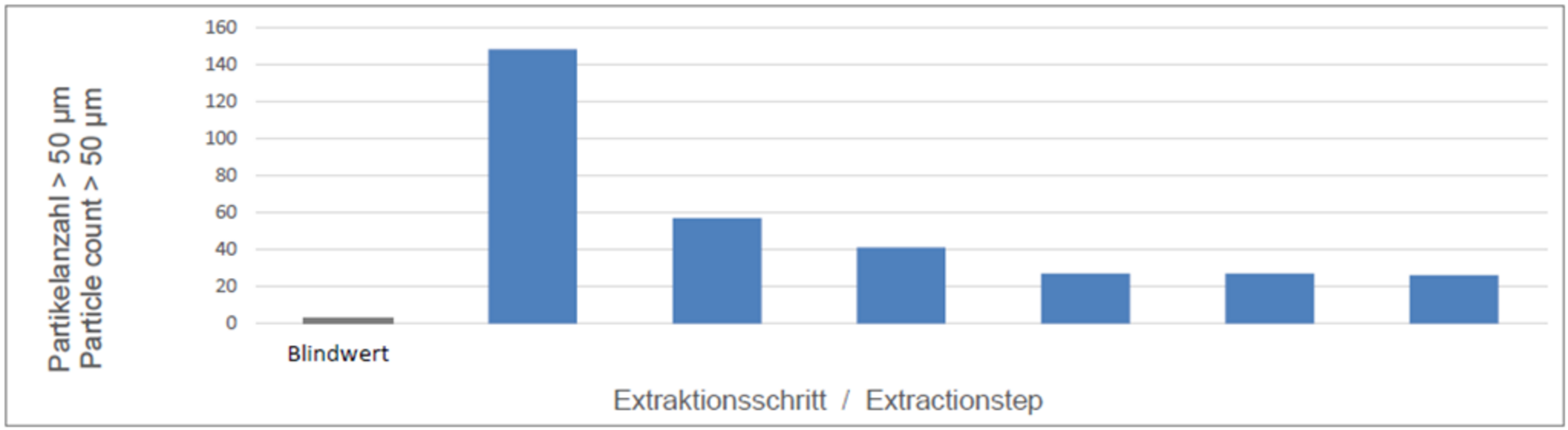
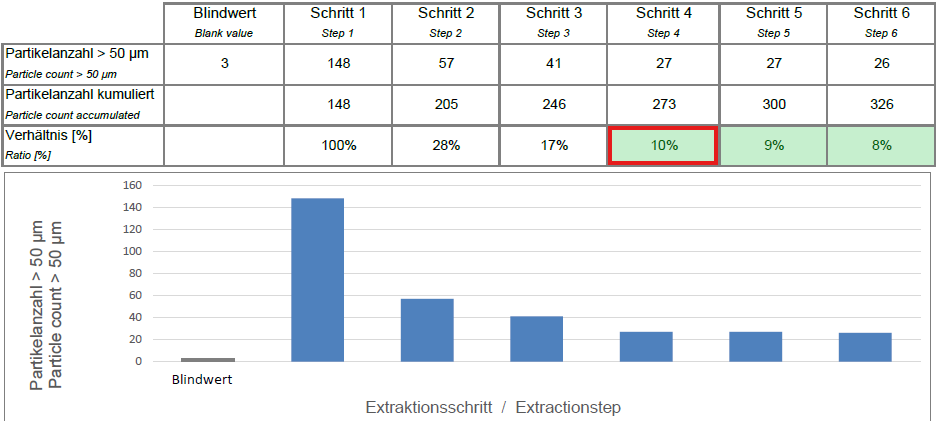
Decay measurement is an experimental method that uses repeated measurements with specified parameters to determine the effectiveness of the extraction using a decay curve. The final test parameters for particle extraction are determined on this basis.
Procedure:
- Preparation: A blank value is generated to qualify the extraction cabinet without a component.
- Sampling: The test lot is sampled six times in succession using the selected extraction method and the specified parameters. The conditions (pressure, solvent quantity, exposure time) remain constant.
- Analysis: After each sampling, the detached particles are collected in a filter membrane and analyzed microscopically.
Interpretation:
- The amount of particles should decrease steadily after each sampling. The decay curve shows a graphical representation of the results.
- The decay curve is used to interpret the effectiveness and corrections of the extraction procedure and its parameters in the event of deviating decay behavior (delayed decay, no decay, increase)
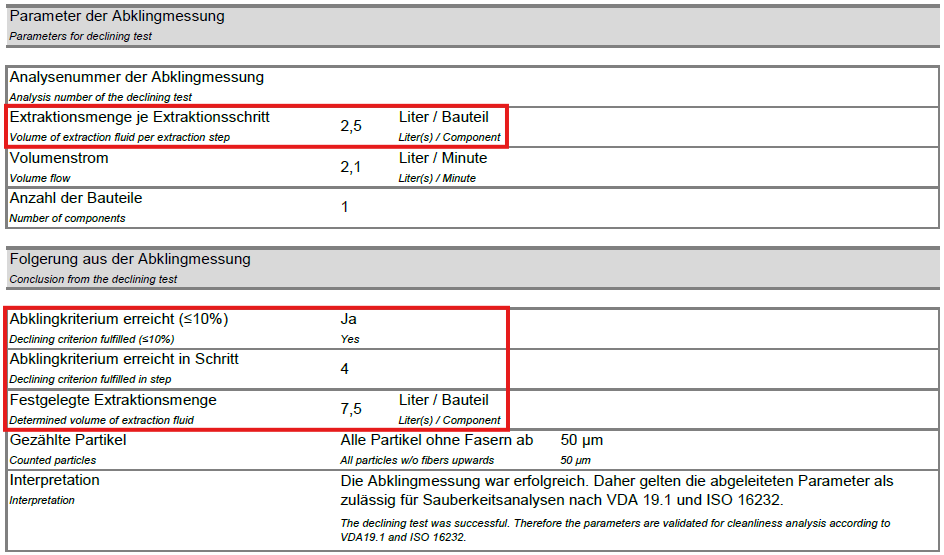
- The ratio of the particle quantity of the last sampling to the sum of the previous samplings should be ≤ 10%. If this is not the case, further sampling is required.
Scope of application:
The decay measurement is used to develop and define effective and valid test conditions for the subsequent routine tests. This ensures comparable results of the routine tests of a component. The decay measurement is fundamentally required for recognized and valid documentation of the cleanliness test.
Double inspection
VDA 19.1 also contains an optional double inspection that can be used to check the effectiveness of a qualified routine procedure. This can be additionally required by various company standards for cleanliness tests and then represents an additional verification of the decay measurement.
For documented cleanliness tests, however, double inspection also opens up the possibility of checking the effectiveness of qualified parameters derived from similar components on geometrically and materially similar components. In addition, the double inspection can also be used to check the effectiveness of test parameters based on empirical values, but only for internal cleanliness tests, e.g. for comparative measurements in cleaning processes.
Procedure:
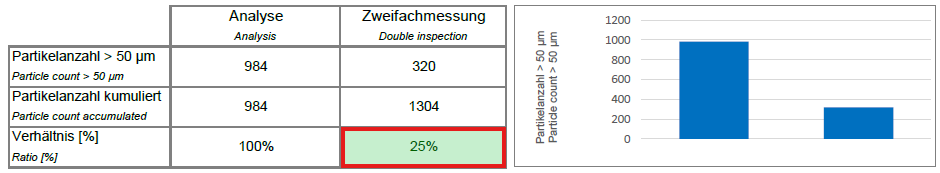
- Preparation: The routine sampling procedure described in the previous section is carried out twice on another inspection lot that has not yet been sampled.
- Measurement: The cleanliness values C1 and C2 are determined.
- Analysis: The results of the two measurements are compared in order to check the suitability of the routine sampling parameters.
Interpretation:
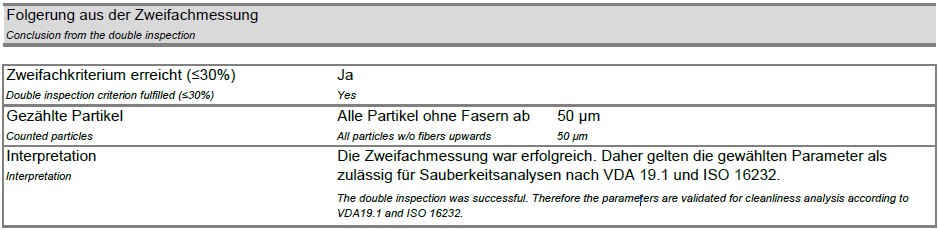
- The second cleanliness value (C2) should be less than or equal to 30% of the sum of the two cleanliness values (C1 + C2).
- If this criterion is met, the parameters developed for routine sampling are suitable and can be defined as a test specification.
- If the criterion is not met, the test parameters must be adjusted.
Areas of application:
- Verification of routine sampling:
The double inspection for checking routine sampling ensures that the test parameters are reliable and effective. It therefore assumes that test parameters have already been determined or could be derived from similar components. For recognized documented cleanliness tests, a decay measurement has therefore already been carried out on the same or a very similar component.
- Verification of assumed test parameters:
As part of product or process development, it can be useful to measure the cleanliness condition of the component initially or as a comparable series of measurements. This can be relevant for the development of cleaning processes, for example, by determining the state of cleanliness before and after cleaning. For this purpose, it may be sufficient to derive test parameters from empirical values and verify them with the double inspection.
Conclusion
Both methods have their specific functions and applications. The decay measurement is required to determine and validate the test parameters for the extraction process, while the double inspection only checks the effectiveness of the existing test parameters.
The decay measurement is the valid basis for the test specification and is therefore the relevant qualification protocol. In conjunction with the test protocol, the test specification and the recorded qualification, this forms the complete and recognized documentation of the cleanliness test.
With only two extraction steps, for which the routine test can ideally also be used, the double inspection is much more cost-effective than the six-fold decay measurement. It is therefore the most economical method for verifying existing test parameters. However, without prior decay measurement, unless it has been derived from similar components or component groups, it is generally not a valid basis for standard-compliant documentation of the cleanliness inspection.
Newsletter registration